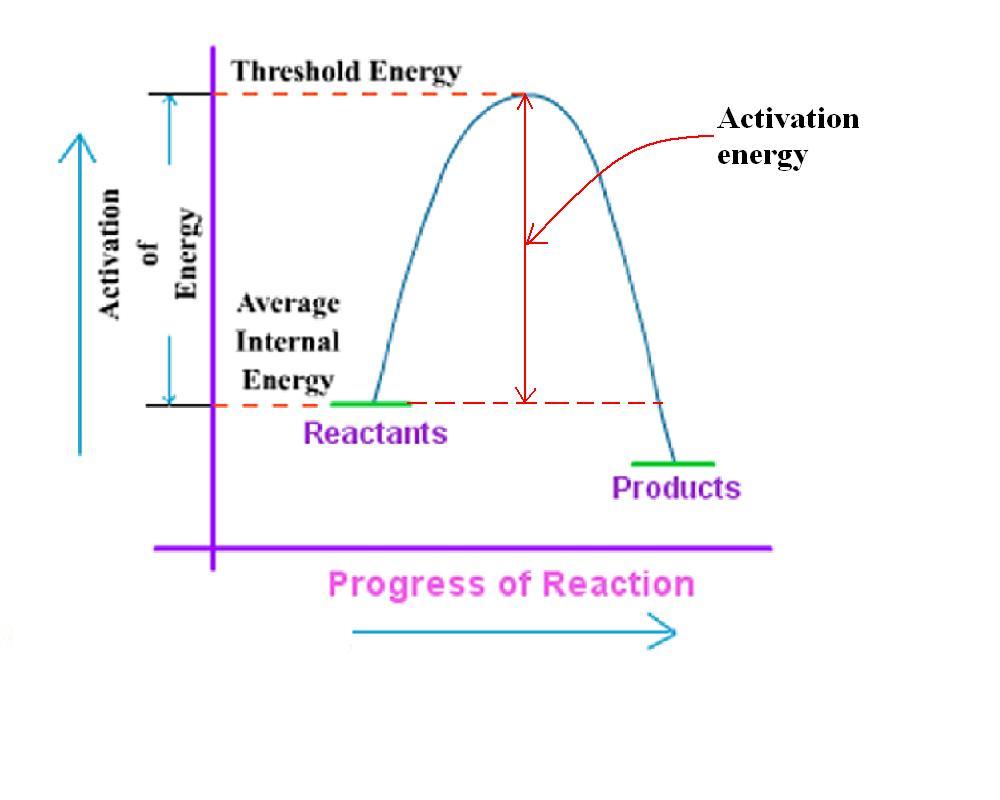
Threshold Energy Example . for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. X + x → y + y. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. We can use the law of conservation of energy and momentum to.
from exogobhlj.blob.core.windows.net
We can use the law of conservation of energy and momentum to. X + x → y + y. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the.
Threshold Energy For Proton at Irene Browning blog
Threshold Energy Example X + x → y + y. We can use the law of conservation of energy and momentum to. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. X + x → y + y.
From www.researchgate.net
An example of the threshold energy determination for aC sample Threshold Energy Example We can use the law of conservation of energy and momentum to. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. X + x → y + y. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon. Threshold Energy Example.
From www.youtube.com
The threshold wavelength for emission of electrons from a given metal Threshold Energy Example calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. We can use the law of conservation of energy and momentum to. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. threshold energy is the minimum. Threshold Energy Example.
From www.youtube.com
Explain Q Value and, Threshold energy Nuclear Chemistry Physical Threshold Energy Example We can use the law of conservation of energy and momentum to. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. X + x → y + y. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon. Threshold Energy Example.
From www.edrawmax.com
What is an Energy Pyramid Diagram EdrawMax Online Threshold Energy Example X + x → y + y. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. threshold energy is the minimum kinetic energy the molecules must. Threshold Energy Example.
From present5.com
Scattering and diffraction Based on chapert 4 Threshold Energy Example for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. X + x → y + y. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. We can use the law of conservation. Threshold Energy Example.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Example threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon. Threshold Energy Example.
From www.slideserve.com
PPT CHEM 312 Lecture 9 Nuclear Reactions PowerPoint Presentation Threshold Energy Example X + x → y + y. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. We can use the law of conservation of energy. Threshold Energy Example.
From www.britannica.com
Energy flow biology Britannica Threshold Energy Example threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. X + x → y + y. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. We can use the law of conservation. Threshold Energy Example.
From www.researchgate.net
The predicted integrated flux above the threshold energy E γ , with an Threshold Energy Example calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. We can use the law of conservation of energy and momentum to. X + x → y +. Threshold Energy Example.
From www.youtube.com
(English) The threshold frequency v0 for a metal is 7.0 X 10^14 s1 Threshold Energy Example calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. We can use the law of conservation of energy and momentum to. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. for any frequency of light above the threshold. Threshold Energy Example.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Energy Example X + x → y + y. We can use the law of conservation of energy and momentum to. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the. Threshold Energy Example.
From www.researchgate.net
Critical energy threshold Download Scientific Diagram Threshold Energy Example for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. X + x → y + y. We can use the law of conservation of energy and. Threshold Energy Example.
From www.researchgate.net
The threshold energy E 0 is determined by the equation (3.1). The red Threshold Energy Example calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. X + x → y + y. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific. Threshold Energy Example.
From www.researchgate.net
Threshold energy model with effectively smaller receptive fields (RFs Threshold Energy Example threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which. Threshold Energy Example.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Example X + x → y + y. We can use the law of conservation of energy and momentum to. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase. Threshold Energy Example.
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy Example We can use the law of conservation of energy and momentum to. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. threshold energy is the minimum. Threshold Energy Example.
From www.youtube.com
Activation energy/threshold energy /activation energy in chemical Threshold Energy Example threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which. Threshold Energy Example.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Example threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. for any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or. Threshold Energy Example.